
In his keynote address to the 2024 Global Specialty Lens Symposium, Professor Emeritus Nathan Efron explained the relevance of the inflammatory nature of contact lens wear to specialty lens fitting.
I AM HONORED to be invited to deliver the 2024 GSLS Keynote Address, and would like to take this opportunity to recount evidence of the intrinsically inflammatory nature of contact lens wear—termed “para-inflammation”—and to explain the relevance of this to specialty lens fitting.
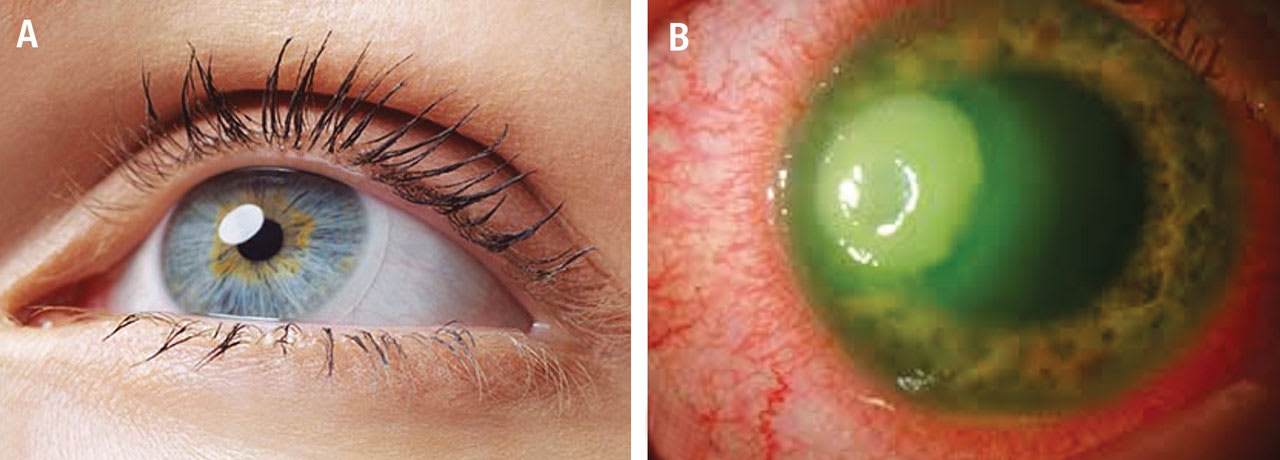
At the outset, it is important to understand that the para-inflammation referred to here is a subtle, subclinical state of inflammation in a normal, healthy, largely asymptomatic contact lens wearer with clear, white eyes (Figure 1A). This discussion does not concern the more severe forms of contact lens-induced keratitis associated with intense eye redness, severe pain, and corneal ulceration (Figure 1B).
The idea that the eye in normal, uncomplicated contact lens wear is constantly in a state of mild inflammation can be traced back to the very first paper published on contact lenses by Adolf Fick in 1888 (Figure 2). I found this after translating and forensically examining his original writings.1

Fick observed a number of responses to contact lens wear, such as conjunctival redness and discomfort, and when closely examining the cornea, he noted, “...in the cornea itself, the well-known inflammatory infiltration with round cells was wholly absent....” The nature of the “round cells” that Fick was looking for is unclear; nevertheless, his failure to observe these “round cells” resulted in Fick dismissing the notion that contact lens wear was inflammatory. Notwithstanding this dismissal, Fick must be credited as being the first to contemplate an inflammatory component of contact lens wear.
Numerous researchers have continued to explore the ocular response to contact lens wear since Fick’s initial observation 136 years ago, including reactions that at least in part would lead to the ultimate conclusion that contact lens wear is inflammatory. However, up until very recently, no one seemed to have “joined the dots” to test this idea.
A PERSONAL JOURNEY OF ENQUIRY
I have been fascinated by the relationship between contact lens wear and inflammation throughout my career, and was perhaps the first researcher since Fick to directly articulate this idea in a paper I published in 1985 titled “Is Contact Lens-Induced Corneal Oedema Inflammatory?”2 Contact lens-induced edema was attributed to hypoxia, but it was not possible to explain corneal edema, or indeed most other adverse reactions to contact lens wear, by this single etiological factor.
Inflammation seemed to be an obvious candidate. I actually wrote the 1985 paper as a follow-up to an experiment I conducted in which I failed to reduce contact lens-induced edema in a group of human subjects using the anti-inflammatory prostaglandin inhibitor Naproxen.3 This failure was probably due to insufficient subject numbers rather than a true lack of effect.
Undaunted by my failed experiment, I continued to contemplate this idea, which I revisited in a 2012 editorial titled “Is contact lens wear inflammatory?”4 After further thought, I finally came to a conclusion, encapsulated in the title of my 2017 paper “Contact lens wear is intrinsically inflammatory.”5 This paper has been highly cited (73 citations in Scopus) and has initiated intense discussion and debate as to the clinical relevance of contact lens-induced para-inflammation.
DEFINING “INFLAMMATION”
I shall begin by defining the term “inflammation” and shall demonstrate how the well-documented responses to contact lens wear meet the various aspects of this definition. I searched numerous medical dictionaries and noted that they all gave similar definitions of inflammation, with the following being a typical example, highlighting three key components. Inflammation is:
1. A normal pathological process provoked by actual or threat of imminent physical, chemical, or biological injury;
2. Characterized by five classic signs—rubor (redness), calor (heat), tumor (swelling), dolor (pain), and functio laesa (loss of function), all/some of which are noted in inflamed tissue;
3. Mediated by a series of cellular and biochemical reactions in affected blood vessels and adjacent tissues.6
The first component of the above definition essentially relates to the causative agent that initiates a response. In the context of contact lens wear, “physical injury” could be caused directly by a contact lens and “chemical injury” may be attributed to the effects of contact lens care or packaging solutions. “Biological injury” could be lipid- or protein-mediated or result from microorganisms entering the ocular tissues during contact lens wear, although the latter scenario would typically result in an overt inflammatory process that is outside the framework of “trouble-free” lens wear being considered here.
The second component listed above stipulates that all or some of the five cardinal signs of inflammation must be observed. It is interesting that these classical descriptors of inflammation, conceived two millennia ago and originally expressed in Latin, have stood the test of time and are still used in modern definitions of inflammation.
The third component of the definition of inflammation is essentially a contemporary addition; that is, there must be evidence of cellular and biochemical reactions in affected blood vessels and adjacent tissues. The 17th century invention of the microscope and developments in laboratory science over the past half century have facilitated demonstration of cellular and biochemical mediators of inflammation.
A NEW CONCEPT—”PARA-INFLAMMATION”
Relatively recently (in 2008), Medzhitov introduced the concept of “para-inflammation” to reinforce the notion that inflammation can have an important adaptive and protective function.7 He begins by proposing three modes of adaptation and maintenance of tissues, stratified by level of severity: basal (homeostasis), para-inflammation, and inflammation.
In the basal state (least severe), tissues are maintained in a homeostatic state, in many cases with the help of inflammatory cells. In noxious conditions (most severe), tissues undergo stress and can malfunction. If the changes are considerable, then adaptation to the conditions requires massive recruitment of inflammatory cells. This extreme response is termed “inflammation,” which follows infection or tissue damage. Medzhitov proposes an adaptive response that has characteristics that are intermediate between basal and inflammatory states, which he refers to as “para-inflammation.”7
According to Medzhitov, para-inflammatory responses are graded: at one extreme they are close to the basal state, whereas at the other, they start to transition into a full inflammation.7 The induction of a para-inflammatory response does not require overt tissue injury or infection; instead, it is switched on by “tissue malfunction” to restore tissue functionality and homeostasis. If tissue malfunction is present for a sustained period, para-inflammation can become chronic.
According to this model, contact lens wear probably results in the eye remaining in a chronic state of para-inflammation that is close to the basal state, whereby the subtle tissue perturbances caused by the contact lenses result in a subclinical tissue reaction to restore functionality and homeostasis in the affected ocular tissues.
DEMONSTRATING CONTACT LENS-INDUCED PARA-INFLAMMATION
To demonstrate that the eye is in a constant state of para-inflammation during contact lens wear, evidence needs to be presented that establishes that the five cardinal signs of inflammation (as defined in the second part of the definition provided above) are present in a contact lens-wearing eye. Also, the two subclinical signs of cellular and biochemical reactions (as defined in the third part of the definition) need to be shown to exist in contact lens-wearing eyes.
Evidence of the presence of the five clinical factors demonstrating lens-induced para-inflammation is overwhelming, and a detailed account of all the evidence supporting this has been discussed in detail previously5 and will not be repeated here. These factors have been summarized in Table 1, with one example of each factor largely derived from my own research.8-12
Evidence of cellular and biochemical reactions to lens wear has only been revealed using modern laboratory and clinical techniques.13-14 Reagent kits for assaying inflammatory mediators have been commercially available for the past 40 years or so, and have been used by many researchers to demonstrate an upregulation of numerous inflammatory mediators during lens wear.15
However, the elusive “missing link” was the demonstration of mobilization of inflammatory cells. This was solved at the turn of the 21st century with the development and commercialization of the laser-scanning corneal confocal microscope (Figure 3).
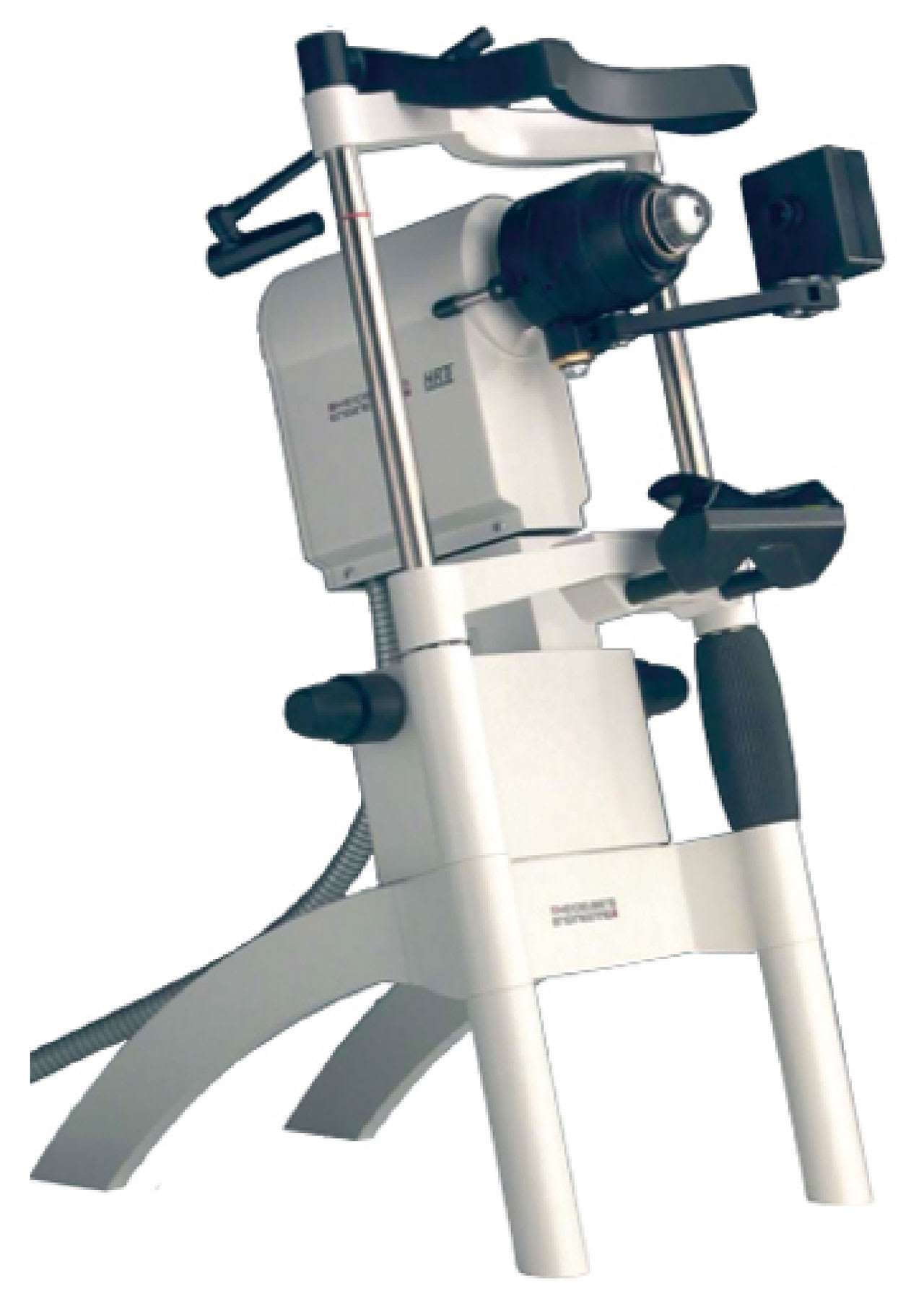
Using this instrument, it is possible to examine the cornea at 700-times magnification, which is a dramatic advance over the slit-lamp biomicroscope that is used for clinical examination the anterior eye—up to a maximum of about 40-times magnification.16 The laser-scanning corneal confocal microscope allows visualization of corneal dendritic cells—presumed to be Langerhans cells. This technique also facilitates quantification of the density, morphology, and movement of Langerhans cells in the cornea and conjunctiva during lens wear. Examples of cellular and biochemical reactions to lens wear are also presented in Table 1.
IS CONTACT LENS-INDUCED PARA-INFLAMMATION PROTECTIVE?
The notion that contact lens-induced para-inflammation is a positive, protective attribute will be counterintuitive to most clinicians; after all, inflammation has a negative connotation, being an entity that is red, hot, swollen, and painful, and limits function. For example, in a medical context, the extensive tissue destruction that occurs in the process of severe microbial keratitis (Figure 1B) can only be viewed as negative.
However, there is a line of thinking that spans back over a century, suggesting that a subclinical or low-grade inflammatory response, which we now term “para-inflammation,” can be beneficial and perhaps protective against more serious inflammation and tissue damage. For example, in 1931, Menkin published a paper titled “Inflammation. A protective mechanism.”17 Chronic immune upregulation of the anterior ocular tissues during contact lens wear (i.e., para-inflammation) is proposed to be a positive, protective phenomenon, whereby upregulation of the immune system, in a non-damaging way, maintains the eye in a state of “heightened alert,” ready to ward off any extrinsic noxious challenge (Figure 4).

Although there is no direct evidence of this protective mechanism, consider the very low incidence of severe microbial keratitis during contact lens wear, estimated to be about 4 cases per 10,000 lens wearers per year.18 Given the constant intrinsic and extrinsic challenges to the eye during lens wear, such as the introduction of potentially noxious microorganisms from fingers during lens application, environmental pollutants, etc., it is remarkable that the incidence of severe microbial keratitis is so low. I would surmise that, in the absence of protective para-inflammation, this incidence value could be as high as 100 cases per 10,000 lens wearers per year, or even higher.
There now seems to be universal acceptance of the notion that contact lens wear is intrinsically inflammatory; however, there is some disagreement as to whether this para-inflammation is truly protective. For example, in their study of the role of corneal TRPA1 and TPRV1 ion channels in contact lens-induced para-inflammation, Datta and colleagues19 concluded that “The significance of corneal para-inflammation during contact lens wear is yet to be determined. Although a primarily protective role has been postulated by Efron, the possibility that it could also enhance tissue susceptibility to infection and other adverse events cannot be excluded.” Further research will be required to determine the extent, if any, of a protective role of para-inflammation during contact lens wear.
IMPLICATIONS FOR SPECIALTY LENS FITTING
To facilitate modeling the inflammatory response to contact lens wear—in particular as it relates to specialty lens fitting—I would extend Medzhitov’s theory7 to portray these responses as a continuous spectrum of inflammation, rather than three discrete states of normal homeostasis, para-inflammation, and serious infection. In this “continuous inflammatory spectrum” model, para-inflammation is the intermediate zone of the spectrum.
To afford protection to the cornea, I propose that a key aim of contact lens fitting should be to keep the eye-lens system in the “sweet zone,” which is at the low-severity part of the para-inflammatory phase of the inflammatory spectrum. This “continuous inflammatory spectrum” model is illustrated in Figure 5, and shall be used as a template for considering inflammatory status during specialty lens fitting.
I have yet to find a clear definition of specialty lens fitting, so I shall attempt to derive my own definition, as follows: “Specialty lens fitting is the fitting of any form of contact lens, as deemed appropriate, to an eye with especially challenging optical, refractive, anatomical, physiological, or pathological characteristics.”
In many respects, therefore, specialty lens fitting is more about the eye than the lens. In any case, the above definition will be used to develop a model of para-inflammation in specialty lens fitting by separately considering the lens, then the eye, and then competing influences of the eye and lens.
The Lens Although specialty lens practice generally has a connotation of fitting a highly sophisticated lens, such as a custom-designed scleral lens, any type of lens may be used depending on the eye being fitted, including daily disposable soft, reusable soft, corneal, scleral, corneo-scleral, and hybrid lenses. Research investigating inflammatory cells and biochemical markers during lens wear has revealed differing levels of inflammatory upregulation with different lens types.14,20-22 These responses are shown schematically in the continuous inflammatory spectrum template in Figure 6, together with some hypothesized estimates.

Figure 6 indicates a possible spectrum of para-inflammation with different lens types, ranging from a very low level for daily disposable soft lenses to moderate para-inflammation with a corneo-scleral lens. All of these responses are hypothesized to be at the low severity end of the “sweet zone” within the inflammatory spectrum.
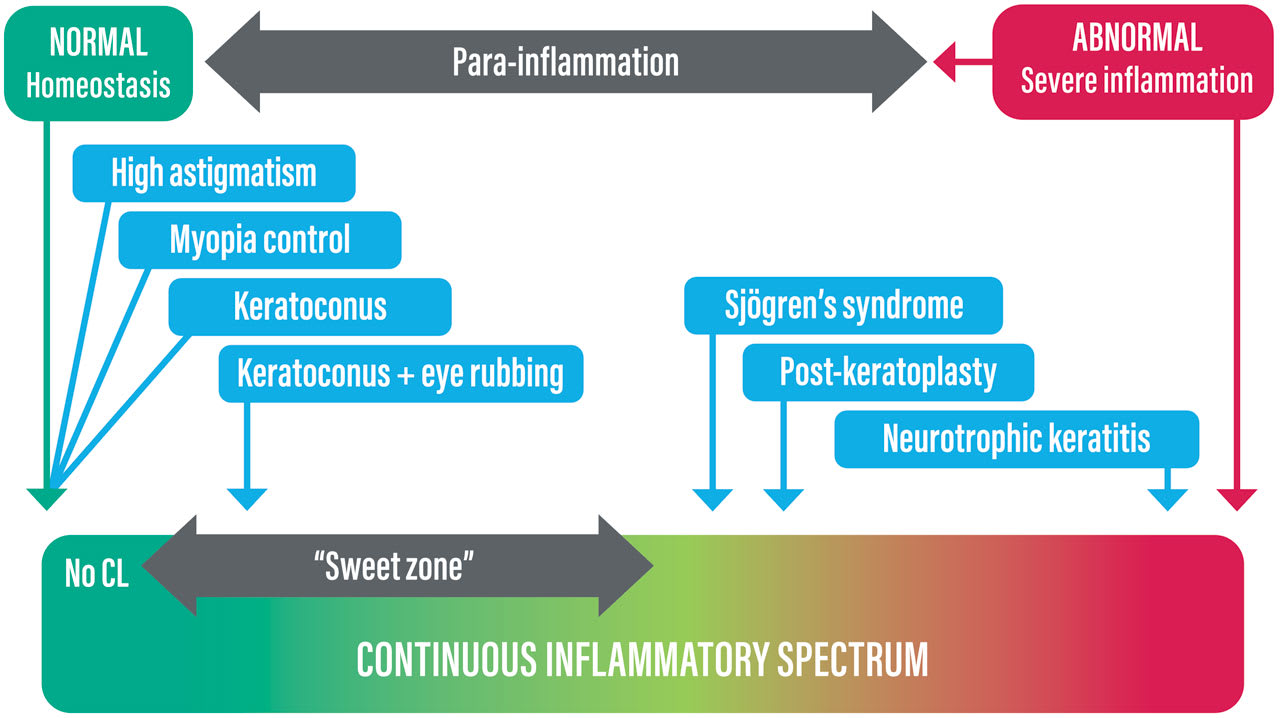
The Eye In considering the eye in specialty lens fitting, a useful starting point in the context of the model being developed here is to consider whether the eye is in an inflammatory or noninflammatory state. An inflammatory state would be assumed in cases such as Sjögren’s syndrome, neurotrophic keratitis, or fitting immediately post-surgery. In each of these examples, the level of inflammation present will be commensurate with—and indeed defined by—the severity of the condition, or in the case of postsurgical fitting, the invasiveness of the surgery and the time elapsed since surgery. A noninflammatory state would be assumed in purely refractive cases such as irregular astigmatism, high myopia, or fitting for myopia control.
One difficulty faced by specialty lens fitters is that there is disagreement in the literature as to whether certain conditions have an inflammatory basis. A classic example is keratoconus, which is claimed by some to be inflammatory in nature,23 whereas others dispute this.24 Uncertainty as to the inflammatory status of a condition being managed with specialty contact lenses may confound consideration of the competing influences of the lens and eye, as discussed below.
The level of inflammation of various examples of inflammatory and noninflammatory conditions that may fall within the remit of specialty lens fitting is illustrated schematically on the inflammatory spectrum template in Figure 7.
Competing Influences As alluded to above, the aim of specialty lens fitting in respect of inflammatory considerations is to “position” the eye-lens system in the “sweet zone,” which essentially represents a low level of para-inflammation. At least from a theoretical standpoint, this involves considering the competing influences of: 1) an increased para-inflammatory response due to the lens, 2) the inflammatory status of the eye at the time of fitting, and 3) the potential therapeutic benefit of the fitting approach.
It is not possible to consider here all of the possible conditions that could be subject to specialty lens fitting, so one example is provided to illustrate the concept of competing influences. Consider a patient with moderately severe Sjögren’s25 being fitted with a scleral contact lens (Figure 8). The starting inflammatory status of the eye is indicated as being in the yellow part of the spectrum, well outside the sweet zone. Placing the scleral lens on the eye will result in an increase in inflammation (short brown arrow pointing right).

However, it would be expected that the therapeutic benefit of the lens, by way of shielding and protecting the ocular surface and maintaining a fluid reservoir between the lens and cornea, would be an overriding influence (long green arrow pointing left), resulting in a lessening of the inflammatory status of the eye-lens system, hopefully approaching—and ideally ending up within—the sweet zone.
STRATEGIES FOR MINIMIZING CONTACT LENS-INDUCED PARA-INFLAMMATION
Specialty lens fitters have a range of options for reducing contact lens-induced para-inflammation. An obvious starting point is to fit a lens type that is known to induce minimal para-inflammation. However, in many cases, lens choice will likely be constrained by the nature of the condition being treated (e.g., a daily disposable soft lens, which induces the least amount of para-inflammation, normally would be unsuitable for moderate to severe keratoconus).
Inflammation can be reduced by fitting lenses with anti-inflammatory coatings that are either natural (e.g., lysozyme)26 or artificially applied (e.g., interleukin-4).27 Soft lenses are now available that provide slow release of anti-inflammatory agents.28 Anti-inflammatory ocular or systemic medications may be prescribed, and patients can be advised to modify their diets and/or take anti-inflammatory supplements such as omega-3 fatty acids.29
CONCLUSION
It is now universally accepted that contact lens wear is intrinsically inflammatory; this low-level, subclinical inflammatory response is termed “para-inflammation.” Para-inflammation is postulated to be a positive, protective phenomenon, whereby upregulation of the immune system maintains the eye in a state of readiness, ready to resist any external challenge. However, some suggest that para-inflammation is not necessarily always protective.
In the context of the inflammatory spectrum described here, specialty contact lens fitters are encouraged to consider the competing influences of the type of lens being fitted, the status of the eye being treated, and the potential therapeutic benefit of the treatment being applied. This theoretical construct may provide a useful framework for managing specialty lens fits, and more broadly, point toward the development of lens designs or other engineering or pharmacological strategies to modulate contact lens-induced inflammation so as to render lens wear safer and more comfortable.
REFERENCES
1. Efron N, Pearson RM. Centenary celebration of Fick’s Eine Contactbrille. Arch Ophthalmol. 1988 Oct;106:1370-1377.
2. Efron N. Is contact lens induced corneal oedema inflammatory? Aust J Optom. 1985;68(5):167-172.
3. Efron N, Holden BA, Vannas A. Effect of the prostaglandin inhibitor naproxen on the corneal swelling response to hydrogel contact lens wear. Acta Ophthalmol (Copenh). 1984 Oct;62:746-752.
4. Efron N. Is contact lens wear inflammatory? Br J Ophthalmol. 2012 Dec;96:1447-1448.
5. Efron N. Contact lens wear is intrinsically inflammatory. Clin Exp Optom. 2017 Jan;100:3-19.
6. Mooney J. Illustrated Dictionary of Podiatry and Foot Science. Edinburgh: Churchill Livingstone Elsevier, 2009.
7. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008 Jul 24;454:428-435.
8. Maldonado-Codina C, Morgan PB, Schnider CM, Efron N. Short-term physiologic response in neophyte subjects fitted with hydrogel and silicone hydrogel contact lenses. Optom Vis Sci. 2004 Dec;81:911-921.
9. Purslow C, Wolffsohn JS, Santodomingo-Rubido J. The effect of contact lens wear on dynamic ocular surface temperature. Cont Lens Anterior Eye. 2005 Mar;28:9-36.
10. Morgan PB, Brennan NA, Maldonado-Codina C, Quhill W, Rashid K, Efron N. Central and peripheral oxygen transmissibility thresholds to avoid corneal swelling during open eye soft contact lens wear. J Biomed Mater Res B Appl Biomater. 2010 Feb;92:361-365.
11. Papas E, Tilia D, McNally J, Lazon de la Jara P. Ocular discomfort responses after short periods of contact lens wear. Optom Vis Sci. 2015 Jun;92:665-670.
12. Dumbleton K, Woods CA, Jones LW, Fonn D. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens. 2013 Jan;39:93-99.
13. Alzahrani Y, Pritchard N, Efron N. Changes in corneal Langerhans cell density during the first few hours of contact lens wear. Contact Lens Ant Eye. 2016 Aug;39:307-310.
14. Kallinikos P, Morgan P, Efron N. Assessment of stromal keratocytes and tear film inflammatory mediators during extended wear of contact lenses. Cornea. 2006 Jan;25:1-10.
15. Deng M, Li M, Liu L, Shi Y, Sun L, Ma X, Zou J. Proteomic profiling of human corneal stroma from long-term contact lens wearers reveals activation of inflammatory responses. Contact Lens Ant Eye. 2023 Jun;46:101820.
16. Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007 Jul;26:398-436.
17. Menkin V. Inflammation: A protective mechanism. Arch Int Med. 1931 Aug;48:249-261.
18. Efron N, Morgan PB. Rethinking contact lens associated keratitis. Clin Exp Optom. 2006 Sep;89:280-298.
19. Datta A, Lee JH, Flandrin O, et al. TRPA1 and TPRV1 ion channels are required for contact lens-induced corneal parainflammation and can modulate levels of resident corneal immune cells. Invest Ophthalmol Vis Sci. 2023 Aug 1;64:21.
20. Saliman NH, Morgan PB, MacDonald AS, et al. Subclinical inflammation of the ocular surface in soft contact lens wear. Cornea. 2020 Feb;39:146-154.
21. Saliman NH, Maldonado-Codina C, Morgan PB. Effect of material and care system combination on subclinical inflammation of the ocular surface in soft contact lens wear. Cont Lens Anterior Eye. 2022 Aug;45:101489.
22. Tse V, Zhou Y, Truong T, et al. Corneal health during three months of scleral lens wear. Optom Vis Sci. 2020 Sep;97:676-682.
23. McMonnies CW. Inflammation and keratoconus. Optom Vis Sci. 2015 Feb;92:e35-e41.
24. Gatzioufas Z, Panos GD, Hamada S. Keratoconus: is it a non-inflammatory disease? Med Hypothesis Discov Innov Ophthalmol. 2017 Spring;6:1-2.
25. Fox RI. Sjögren’s syndrome. Lancet. 2005 Jul;366:321-331.
26. Omali NB, Subbaraman LN, Coles-Brennan C, Fadii Z, Jones LW. Biological and clinical implications of lysozyme deposition on soft contact lenses. Optom Vis Sci. 2015 Jul;92:750-757.
27. Jhanji V, Nolfi A, Kulkarni M, Brown BN. Contact lens delivery of interleukin-4 for treatment of dry eye disease promotes anti-inflammatory macrophage population. ARVO 2020. Abstract #1959.
28. Tieppo A, Pate KM, Byrne ME. In vitro controlled release of an anti-inflammatory from daily disposable therapeutic contact lenses under physiological ocular tear flow. Eur J Pharm Biopharm. 2012 May;81:170-177.
29. Bhargava R, Kumar P. Oral omega-3 fatty acid treatment for dry eye in contact lens wearers. Cornea. 2015 Apr;34:413-420.




